SPEAKERS
2026 -Select:
PMWC 2026 PIONEER HONOREE
Ranked #2 Most Influential Person in Healthcare in 2024
PMWC 2026 PIONEER HONOREE
*Nobel Laureate
*2024 Breakthrough Prize Laureate (Life Sciences)
President & CEO of Stanford Health Care
PMWC 2026 PIONEER HONOREE
*Lasker “America’s Nobels” & Gairdner Laureate
Panel: 25+ Years of the Human Genome — What Made It to the Bedside
Led the First Human Genome Sequencing
Pioneered automated DNA sequencing and systems biology
Pioneered Entrepreneurship to Advance Genomics
HONOREES
2025 -Select:
PMWC 2025 Luminary HONOREE,
NOBEL LAUREATE
PMWC 2025 LUMINARY HONOREE, CEO & FOUNDER, NVIDIA
15-MINUTE PRESENTATIONS
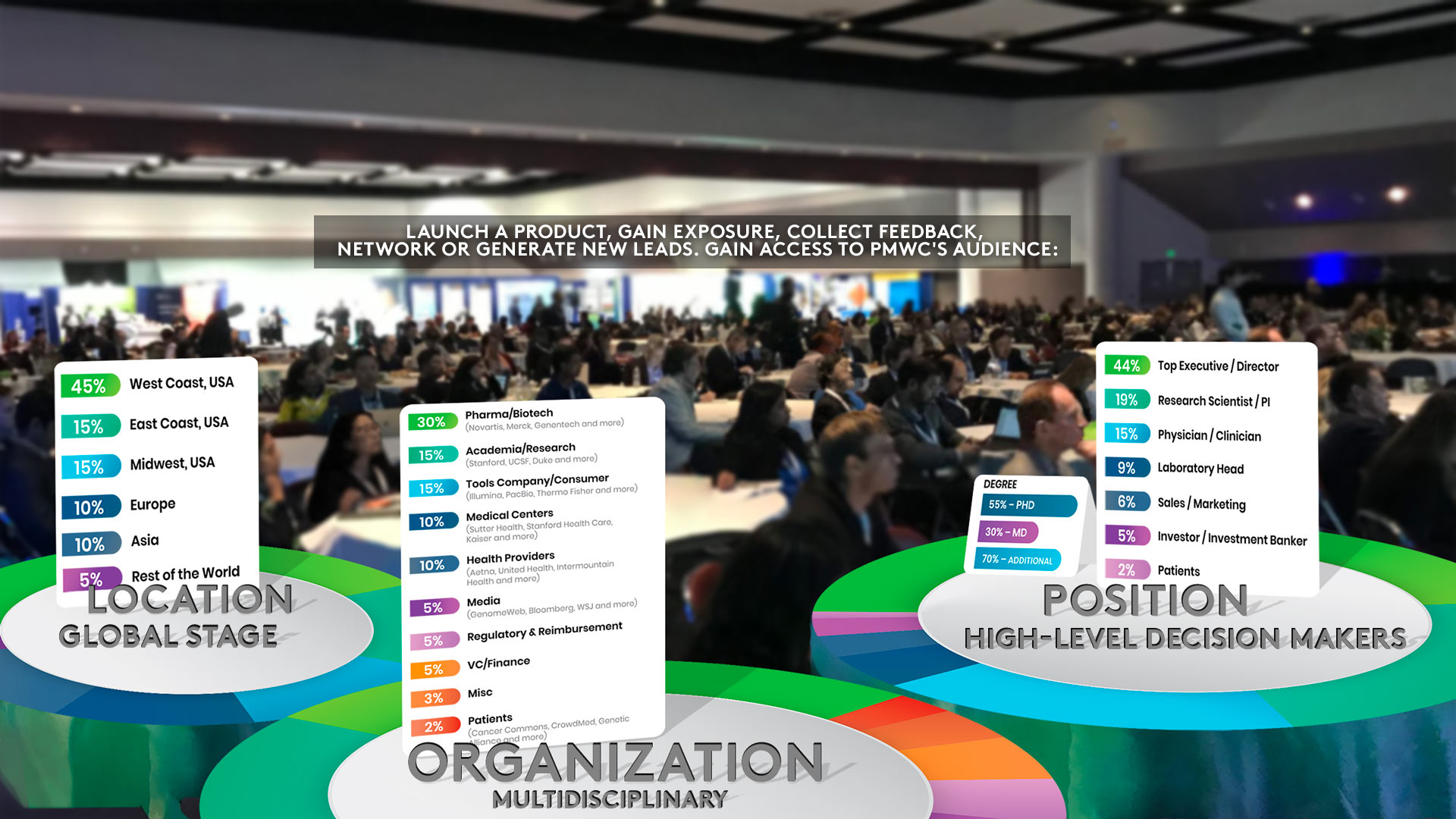
AUDIENCE: UP TO 200 INVESTORS, POTENTIAL CLIENTS AND PARTNERS
Apply by OCT. 16 !
The Foremost Precision Medicine Conference
• Gathering recognized leaders, top global researchers and medical professionals, plus innovators across healthcare and biotechnology sectors
• Showcasing latest practical content that helps close the knowledge gap among different sectors
• Promoting cross-functional fertilization & collaboration to accelerate Precision Medicine
• Main Tracks and Showcases (6 Total) that provide a mix of established and upcoming perspectives
• Luminary and Pioneer Award Ceremony honoring those who transform healthcare by advancing precision medicine in the clinic
PMWC provides a valuable insight for physicians and others who may be wondering how close we are getting to realizing the arrival of personalized medicine. The conferences are helpful in understanding where and how the envelope is being pushed.
Peter Paul Yu, MD, FACP, FASCO, Immediate Past President, ASCO
PMWC has proven, time and time again, that it attracts thought-leaders from all the relevant fields and catalyzes crucial collaboration through inspiring and practical program content. This is the Conference for entrepreneurs to meet payors, and for researchers to connect with service providers and for clinicians to hear from leading providers.
Lee Hood, PhD, MD, President, Institute for Systems Biology

DAYS

ATTENDEES (35 COUNTRIES)

EXHIBITORS

PARALLEL TRACKS
REGISTRATION
Tickets to PMWC - Est. 2009
Loading

PMWC Overview
PMWC, the “Precision Medicine World Conference” is the largest & original annual conference dedicated to precision medicine. PMWC’s mission is to bring together recognized leaders, top global researchers and medical professionals, and innovators across healthcare and biotechnology sectors to showcase practical content that helps close the knowledge gap between different sectors, thereby catalyzing cross-functional fertilization & collaboration in an effort to accelerate the development and spread of precision medicine.
Since 2009, recognized as a vital cornerstone for all constituents of the health care and biotechnology community, PMWC provides an exceptional forum for the exchange of information about the latest advances in technology (e.g. DNA sequencing technology), in clinical implementation (e.g. cancer and beyond), research, and in all aspects related to the regulatory and reimbursement sectors.
Testimonials

Format
The conference format consists of five parallel talks spanning 3 full days. Main Tracks 1-4 include sessions by leaders in the commercial, pharmaceutical, academic, government, regulatory, venture capital, and non-profit arenas that deliver a broad and up-to-date array of content across the various facets of precision medicine. Session discussions focus on time-relevant aspects with a selected set of key stakeholders, while commercial sessions cover the latest developments in technologies that are instrumental for the success of further adoption of precision medicine.
Additional 2 Tracks, feature Showcases: companies and research institutions can promote their platforms, launch products, and share research developments to a targeted audience – Apply.
For over a decade, PMWC has recognized individuals who have played a significant role in transforming health care by advancing precision medicine in the clinic with the Luminary and Pioneer Awards. The honorees’ numerous technological and scientific contributions have expedited this transformation as demonstrated by the clinical adoption of precision medicine, and the ongoing introductions of novel clinical applications. For a deeper look into the fascinating achievements of our past awardees see the awards page.
Receive the latest news about the field of precision medicine, and the conference from Tal Behar, PMWC’s President:
Levi Garraway’s responses to interview questions from Tal Behar, Precision World Medicine Conference
1. IO focus: If you could green-light one immuno-oncology combination for a definitive Phase 3 in 2026, which patients benefit, and what one biomarker makes or breaks it?
In solid tumors, it seems as though we may be entering an era where IO/ADC combinations could have benefit across multiple indications. Therefore, I’d like to see a “next-gen” immuno-oncology asset combined with a “next-gen” ADC in (IO-responsive) solid tumors enriched for ADC target expression. Beyond that simple tissue biomarker, I would love to see our machine learning colleagues bring forth new and generalizable imaging “biomarker” algorithms – or possibly multimodal imaging/genetic algorithms – that can help discern up front who is most likely to benefit from IO-based therapy, and who is unlikely to benefit. With such information in hand, patients could increasingly be shifted to other therapeutic options or clinical trials without having to wait until formal treatment failure (oftentimes, clinical progression brings new complications which in turn limit future therapeutic choices… so pivoting early where possible could make the difference for many patients).
2. Beyond IO: Beyond CAR-T, which single modality or precision therapy will move the OS needle in solid tumors in the next 3–5 years, and what single trial readout or biomarker is your litmus test for success?
Here, I’ll call out bispecific antibodies that include an IO component – and I’ll also cheat a bit by pointing out that this modality is quickly evolving to include trispecific moieties. We’ve already seen the transformative potential of next-generation bispecific antibodies across solid tumors and hematologic malignancies. As trispecific variations emerge, this impact should only continue to grow. As much as I enjoy genetic and molecular biomarkers, what I love most is seeing deep responses on imaging; e.g., impressive “partial responses” (PRs) in solid tumors, or numerous “very good partial responses” (VGPRs) in hematologic malignancies – and of course, complete responses (CRs) in all instances. These almost always translate into meaningful progression-free survival and overall survival, and seeing those types of patient results just never gets old.
3. AI with receipts, not buzzwords:
Where is Roche already using AI/gen-AI in product development (e.g., de-novo design, trial design/patient selection via clinico-genomics, digital pathology)? What hard KPIs prove impact (hit rate ↑, cycle time ↓, success probability ↑), and what’s the next 12-month expansion?
Two things are true: (1) It is hard to find a segment of the R&D continuum that is not being augmented by AI in some way; and (2) We are still only at the very beginning of our AI journey. (So we certainly don’t have all of the receipts yet!)
Specifically in our product development organization, AI/ML approaches are starting to play a major role in improving critical decisions made throughout the process of clinical trial design and execution. This includes indication selection, defining endpoints, inclusion and exclusion criteria, optimizing patient risk stratification, enhancing the PTS of a clinical program, and monitoring of safety-related data as the trial proceeds, to name a few. Here, we leverage structured data, data from previous trials and real-world evidence (RWD), including imaging or multimodal data. This also can enable “digital twins” to serve as comparator arms in internal trials for decision-making, or even reducing the number of participants needed.
We are already starting to see evidence that AI-driven systems can reduce human error and operational risk while improving oversight. Generative AI can support the creation of essential clinical trial documents, such as protocols, statistical analysis plans (SAPs), clinical study reports (CSRs), and assist in generating statistical code for programming tasks. Finally, we expect agentic AI to enable a further transformation across product development by augmenting a wide range of workflows. Together, these ongoing efforts should markedly increase the innovativeness, efficiency, consistency, and accuracy of our late-stage R&D engine.
4. IO reality check (beyond slogans):
Which combinations or “checkpoint 2.0” strategies (e.g., PD-(L)1 + anti-VEGF, costims, cytokines, TIGIT, etc.) are showing durable, clinically meaningful benefit in solid tumors? What biomarkers (ctDNA/MRD, pCR/MPR, spatial profiling) will Roche insist on to make them stick in practice?
As a general rule, emerging IO therapies that have shown particular promise are often those that can help cytotoxic T-cells do their job even better (as opposed to those that act on other types of cells or factors in the immune microenvironment). Several bispecific antibodies and novel therapeutic combinations that have shown promise in recent clinical studies arguably are working through that mechanism. At the same time, we have to be honest: many immunotherapy hypotheses that seemed promising in the lab didn’t pan out clinically; and, conversely, there are now examples that show high clinical promise that were not necessarily predicted in preclinical studies. So, going forward we certainly do need to keep the bar high regarding specific therapeutic hypotheses, including the clinical or molecular sub-populations in whom the mechanism is most likely to work. Ultimately, however, the proof is in the clinic, which usually requires robust single-agent activity nowadays. Without good single-agent activity, it is often necessary to complete fairly large phase 2 studies before you have any real sense of efficacy, and even then, disappointments can still happen in subsequent pivotal trials
5. Where’s the puck really going?
If you had to allocate Roche R&D focus over the next 3–5 years across oncology (incl. IO), cardio-metabolic/obesity, neuroscience, and other precision areas, what moves up or down vs. five years ago—and why? What does that mean for BD/partnerships?
As a large innovative pharmaceutical company, our goal is always to “follow the science”, by which we mean fundamental discoveries that could lead to transformative medicines. In doing so, we try not to be overly biased toward one therapeutic area versus another. Having said that, oncology remains one of the largest areas of unmet need in all of human disease. We need look no farther than the waiting rooms of countless cancer centers around the globe that are crowded with patients who need care. Many of us (myself included) have witnessed the ravages of cancer within our own families or amongst our dearest friends and loved ones. While it’s clearly true that scientific advances are happening in multiple therapeutic areas at an unprecedented scale – and we will invest to bring great medicines across a range of diseases – substantial investment in oncology remains of paramount importance.
- 10 Oct ,2025
Interview with Keith Booher
Showcase Track S1, Day 2 / February 6th: AI and Data Sciences Showcase
1. Your talk emphasizes AI-driven workflows for identifying repurposed drugs. Can you share a specific case where your platform identified a treatment for a rare disease, and how it impacted patient outcomes?”
Yes, absolutely… But first of all, I need to emphasize the highly productive partnership we’ve developed with Unravel Biosciences, our industry partner based in Boston. By coupling Zymo Research’s sample preparation and next-generation sequencing (NGS) workflows with their data analysis platform, we’ve seen huge potential for AI to positively impact patients who may have nowhere else to turn.
As one illustrative example, a pediatric neurologist connected Unravel Biosciences to a patient with a severe and progressive form of KMT2B dystonia living in remote Arizona. Using Unravel’s rareSHIFT https://www.rareshift.org/ program, which provides access to their AI discovery platform, RNA samples from the patient and a healthy control relative were collected using Zymo’s nasal swabs with DNA/RNA Shield https://www.zymoresearch.com/pages/sample-storage-ambient-temperature preservation solution, returned by mail, and sequenced by Zymo. The transcriptome data was analyzed using Unravel’s BioNAV AI software, which identified a new therapeutic mechanism that could be targeted with an existing drug. The patient’s clinician prescribed the drug, and within days the boy showed remarkable improvements: his dystonia symptoms ceased, his sleep normalized (from 30–60 minutes per night to full, restful sleep), his ADHD symptoms dramatically decreased (allowing him to focus on tasks for 5–9 minutes instead of 20–30 seconds), and probably most importantly of all, he recovered significant ability to communicate with his family. He quickly began to understand instructions, which enabled his family to take him to the store and other public places, and after a few months he even started to use sign language as a form of self-expression, all despite having lost the ability to speak 4 years before. Unravel published a video sharing this patient’s story: https://www.youtube.com/watch?v=LmcvbzHQzvM
The company now works on over 40 rare disease programs and has collected more than 1,300 samples, which correspond to hundreds of patients, all using Zymo’s DNA/RNA Shield home collection kits and the jointly developed RNA extraction and NGS workflow. All of the programs where patients have worked with their clinical team to test a drug have led to similar clinical successes. Looking ahead, the company is initiating several clinical trials that will rely on frequent sample collection using Zymo kits as a way to boost their AI dataset and accelerate the discovery of novel disease biology.
2. AI solutions often face challenges in accessibility for smaller labs or institutions. How does Zymo’s workflow democratize access to AI-driven drug discovery, and what steps do you see as critical for broader adoption?
Zymo has developed a robust nucleic acid stabilization solution called DNA/RNA Shield that preserves the genetic integrity and expression profiles of samples at room temperature, which is critical in generating high-quality -omics datasets needed to utilize AI’s full potential. This, together with self-collection methods like nasal/oral swabs or finger prick devices, has enabled companies like Unravel and many other researchers to decentralize sample collection. The ease of self-collection together with being able to ship DNA and RNA around the world at ambient temperature (no cold chain) has unlocked connections between patients, researchers, and clinicians. Patients are no longer required to travel to a center of excellence or even leave their home. This has accelerated drug discovery by companies like Unravel, enabling them to build new databases of primary patient data from highly diverse ethnicities and geographies to fuel their AI pipeline within just a few months. Furthermore, Unravel started the rareSHIFT program precisely to offer other groups and even individuals access to their powerful platform. We see this as a great example of how key innovative technologies—such as Zymo’s self-collection kits and NGS workflows, combined with Unravel’s AI-driven drug discovery platform—can dramatically accelerate drug development and ensure that effective treatment options are available for all patients, no matter how rare their condition or how remote their location.
3. Looking ahead 5-10 years, how do you see AI and multi-omics shaping the future of personalized medicine, particularly in addressing unmet medical needs?
As an example of future possibilities, Unravel has closed the loop between patients and effective treatments using AI and Zymo’s sample collection kits. This process takes only a month or two, enabling patients to work with their clinicians to identify repurposed drug candidates they can test today, while also validating drug targets to build even more effective treatments for tomorrow. We collaborate with Unravel to tackle rare, ultra-rare, and even completely unique N-of-1 disorders across six continents. This represents a paradigm shift in both clinical care and novel drug development for even the rarest and complex of disorders. We envision a future where AI, combined with global sample collection, accelerates the pace of drug discovery by enabling large-scale personalized omics data collection, decentralized clinical trials, and a personalized N-of-1 approach to all disorders, even relatively common disorders like Alzheimer’s, neuropsychiatric disorders, autoimmune diseases, and infectious diseases.
- 09 Jan ,2025
Interview Questions for Mike Snyder
- Can you describe a project or experience where you integrated data from wearables with multi-omics data? What were the main challenges you faced, and how did you address them?
We have several projects running right now where we are integrating smartwatch data, omics data and continuous glucose monitoring (CGM) data to better manage glucose levels. The study predicts which features, omics (including microbiome), and physical activity to better manage people’s glucose levels. It not only helps tell people what to do, but when to do them. For example, people who are insulin resistance get better benefit (in terms of glucose levels) from exercise in the morning whereas those that are insulin sensitive get more benefit from exercise in the afternoon.
- How do you foresee the integration of wearable data and genomic information shaping the future of treatment plans in clinical settings? What are the potential challenges and benefits of implementing such personalized treatment strategies?
It will not just be the integration of wearable data will genomics data—it will be wearable data with all kinds of other data including omics, clinical and lifestyle data. The wearable data is ideal for tracking physiology in real time and combining this with genomics and biochemical data gives a much more complete picture of what is occurring. In our initial deep profiling study of 109 volunteers we found 49 major health discoveries (and minor ones as well) from the combination of all of these data types (Rose et al, 2019).
- What are the key ethical considerations that must be addressed when designing and implementing studies or interventions that utilize wearable technologies and multi-omics data?
We certainly want to be as inclusive as possible and make sure all individuals can benefit from these technologies. Indeed, one nice thing about wearables is they can be used in remote settings and they are relatively inexpensive. Moreover, algorithms can be trained on extensive data from the individual so, in principle, everyone can benefit from personalized health monitoring.
As predictive capabilities of wearable and multi-omics data evolve, how should healthcare providers manage the ethical dilemma of predicting and disclosing potential future health conditions to individuals and their families?
I think the individual should be in control of what information is relayed back to them. That is to say they work with their physician to determine if they want to know both actionable information (e.g. BRCA status) as well as nonactionable information (e.g. Huntington’s status). I think most wearable data is actionable. The individual works with their family to decide what other members might want to know. In general, the omics and wearable information can be used for improving the health of individuals and early detection of disease.
Sources:
- 04 Jan ,2025