SPEAKERS / HONOREES
2026 -Select:
PMWC 2026 PIONEER HONOREE
Ranked #2 Most Influential Person in Healthcare in 2024
PMWC 2026 SPEAKER
*2025 Nobel Laureate
President & CEO of Stanford Health Care
UNEP Entrepreneurial Vision Laureate
Led the First Human Genome Sequencing
Pioneered automated DNA sequencing and systems biology
*2024 Breakthrough Prize Laureate (Life Sciences)

DAYS

ATTENDEES (35 COUNTRIES)

EXHIBITORS

PARALLEL TRACKS
REGISTRATION
Tickets to PMWC - Est. 2009
Loading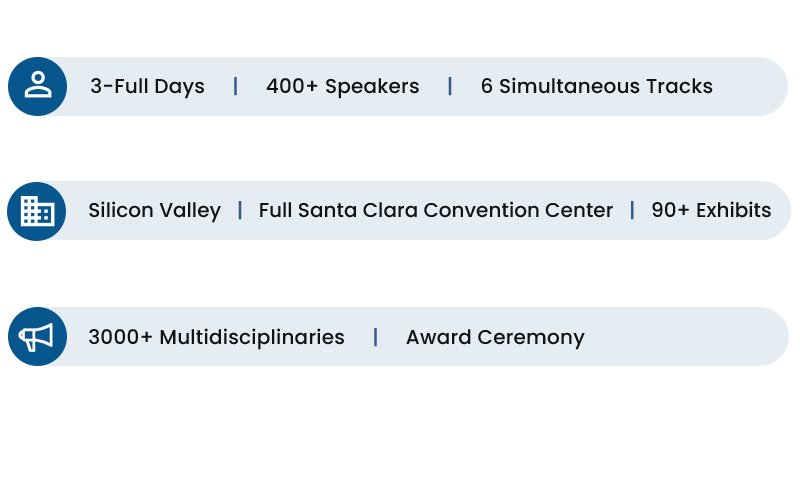


Format
The conference format consists of five parallel talks spanning 3 full days. Main Tracks 1-4 include sessions by leaders in the commercial, pharmaceutical, academic, government, regulatory, venture capital, and non-profit arenas that deliver a broad and up-to-date array of content across the various facets of precision medicine. Session discussions focus on time-relevant aspects with a selected set of key stakeholders, while commercial sessions cover the latest developments in technologies that are instrumental for the success of further adoption of precision medicine.
Additional 2 Tracks, feature Showcases: companies and research institutions can promote their platforms, launch products, and share research developments to a targeted audience – Apply.
For over a decade, PMWC has recognized individuals who have played a significant role in transforming health care by advancing precision medicine in the clinic with the Luminary and Pioneer Awards. The honorees’ numerous technological and scientific contributions have expedited this transformation as demonstrated by the clinical adoption of precision medicine, and the ongoing introductions of novel clinical applications. For a deeper look into the fascinating achievements of our past awardees see the awards page.
Receive the latest news about the field of precision medicine, and the conference from Tal Behar, PMWC’s President:
responses to interview questions from Tal Behar, Precision World Medicine Conference
Professor, University of Toronto; ALLAN SLAIGHT Senior Scientist, Princess Margaret Cancer Centre/University Health Network; Co-Founder and CSO, Adela
- Where does methylation add indispensable value in MRD vs mutation‑only approaches (e.g., low‑shedding tumors, tissue‑of‑origin context, earlier relapse signal), and what prospective study would convince frontline oncologists?
- Which near‑term clinical indication for cfDNA methylome profiling will cross the evidence bar first (high‑risk screening, surveillance after curative therapy, or something else), and what endpoint (earlier stage shift, reduction in unnecessary procedures, survival surrogates) will matter most?
- Looking ahead, what AI or multi‑omic combos (methylation + fragmentomics + mutations/proteins) are most likely to reduce false positives without sacrificing early‑stage sensitivity?
- Where does Adela see the greatest opportunities for its platform across the cancer care continuum?
Adela’s platform is best deployed across a few points on the cancer continuum. Near-term, post-curative MRD, surveillance, and response monitoring are the clearest opportunities: sensitive, tissue-naïve methylome readouts detect response/relapse earlier integrating cleanly into existing follow-up schedules. In addition to therapy monitoring, response prediction will also benefit from methylome signatures that reflect tumor state, not just burden, yielding fast and more informative readouts than imaging alone. In the medium term, risk-adapted screening becomes feasible by combining methylation with AI-based algorithms to achieve the positive predictive value required for responsible population use.
- 12 Nov ,2025
Q1. Single-cell and spatial assays are powering translational research but aren’t yet routine diagnostics. What’s the biggest barrier — scientific, regulatory, or cultural — to bringing these technologies into clinical use?
The biggest barrier right now is clinical evidence. We already know that single-cell and spatial assays can capture critical biology that traditional diagnostics can’t. The technology is maturing – it’s robust, scalable and starting to be used in translational studies that link cell and tissue biology to treatment response in ways that weren’t possible before.
What’s needed next are large, well-powered studies across disease indications that build on the clinical signals already emerging from this initial work. At 10x, we’re working hard to make that possible.
That’s the inflection point for any new measurement technology. Once you can show that what you’re measuring actually predicts patient outcomes and improves clinical decisions, whether that’s providing a prognosis or choosing a therapy, the rest follows. Regulators, clinicians and payers all move quickly if the evidence of clinical utility is there and if that evidence is strong.
So our focus at 10x is enabling that shift: working with researchers to generate that foundation, to run those studies at scale with clinical samples in ways that are reproducible and cost-effective. As that body of evidence builds, the regulatory and cultural alignment will naturally follow.
Q2. As spatial platforms generate unprecedented cellular detail, how do you see AI and multi-omics integration accelerating early detection and treatment response prediction in the next few years?
Progress in AI has been the biggest story in the world over the past several years. Less discussed is the remarkable progress in technologies that measure biology. The two trends are actually incredibly complementary. The newest generations of AI models require relevant large-scale high-quality data. Spatial analysis delivers exactly that. At the same time, spatial analysis stands to benefit from AI’s ability to extract deeper insights from complex data.
The convergence of AI and single cell and spatial technologies connects molecular detail to human health in ways that simply weren’t possible before. As we bring together genomic, spatial and clinical data, we can train AI models to recognize early patterns of disease or predict how patients will respond to treatment.
We’re already seeing this next phase of biology take shape. Across the field, researchers are using 10x tools to build high-resolution biological datasets that power emerging AI models like virtual cells – helping reveal how diseases develop, what happens when those disease cells are perturbed and how we might intervene with more precise therapies. Over the next several years, this convergence will help move precision medicine toward the world of much richer biological data and much greater clarity in clinical decision-making.
Q3. 10x has already transformed how scientists explore tissue biology. Over the next decade, how do you see the company’s role evolving in enabling clinical decision-making and shaping precision medicine?
From the very beginning, we built 10x to measure biology as precisely and robustly as possible – first to enable discovery and ultimately to transform medicine. That next phase is now taking shape, as single-cell and spatial technologies move from the research bench toward clinical applications, much as sequencing did over a decade ago.
Our focus today is on enabling the studies that make that shift possible – large, well-powered translational studies that link cellular biology to clinical outcomes. Those results will define the diagnostics and treatment-selection tools of the future.
Longer term, we see 10x playing a key role in informing patient care. We are continuing to invest in technology capabilities that will make our platforms higher throughput, more integrated, automated and robust for clinical use. We will expand our investment in clinical evidence generation for particularly compelling use cases to accelerate adoption. Over the next decade, our goal is to make single-cell and spatial analysis as essential to understanding disease and guiding its treatment as sequencing is today.
Q4. What moment or discovery in your journey with 10x most clearly showed you the profound impact single-cell and spatial genomics could have on patient care?
There was a poignant story from the early days. A customer used single-cell analysis to look retrospectively at samples from cancer patients who had been treated with immunotherapy. There was a particular patient who had a recurrence and passed away. They couldn’t figure out what happened with the tools available to them at the time. With single-cell, the explanation jumped out very clearly. The researcher told me she wished our tools existed when that patient was still alive, because she would have adjusted the treatment. A small story, but points to so much potential.
Since then, there have been tons of groundbreaking research that reinforces the promise of these technologies to impact patient care. The very first CRISPR-edited cell therapy in humans was monitored using single-cell sequencing, and a recent groundbreaking experiment using cell therapy to cure Lupus was enabled with single-cell as well. Researchers have also used our technologies to map cellular “neighborhoods” in the tumor microenvironment that reveal why some patients respond to therapy and others don’t; map millions of single cells in Alzheimer’s brains, revealing how gene-control systems falter in disease but remain preserved in people who stay cognitively resilient; to uncover the cellular ecosystems that drive glioblastoma progression and therapy resistance; and even to replace painful bone-marrow biopsies with simple, non-invasive blood draws that provide comparable biological insights. Each of these advances is laying the groundwork for what comes next and brings 10x closer to the clinic.
And most recently, in T-ALL, seeing scientists uncover a treatment-resistant cancer cell type using single-cell methods that could change the standard-of-care reinforced in my mind the vision we are building towards: Deep, accurate measurements using our tools leading to better diagnostics and, ultimately, better care.
- 11 Nov ,2025
Founder and Chair, European Liquid Biopsy Society (ELBS)
Director, Institute of Tumor Biology, University Medical Center Hamburg-Eppendorf (UKE)
- Emerging liquid biopsy approaches—such as cfDNA methylation profiling, fragmentomics, and AI-driven analyses—are often described as game-changers for early cancer detection. Which of these innovations do you believe holds the most promise for improving cancer screening, and what challenges must be overcome to translate them into routine clinical practice?
RE: I believe that a combination of these liquid biopsy approaches holds most promise for improving cancer screening and would also include non-ctDNA biomarkers such as circulating extracellular vesicles, proteins, metabolites, RNAs and tumor cells. Such a composite biomarker panel is currently evaluated for early pancreatic cancer screening in the EU Horizon consortium PANCAID (https://pancaid-project.eu; Lead-PI: K. Pantel). Challenges that must be overcome include optimization of pre-analytical variables (e.g., each biomarker may have different optimal conditions for blood tubes), availability of biobanks from patients with pre-neoplastic or early-stage disease with long term follow up, cancer-associated aberration in non-cancer cells of ageing patients, andcost effectiveness as compared to existing screening modalities.
2. You have emphasized focusing on high-risk populations (e.g., genetically predisposed individuals or patients with chronic conditions) for liquid biopsy screening, rather than broad population-wide testing. Given the risks of overdiagnosis, how should researchers decide which cancers and patient groups to target first with early-detection liquid biopsy?
RE: Key is in my opinion the clinical need such as a tumor types that are notoriously detected at late stages and where no routine screening exists yet. For some tumor types such as pancreatic or ovarian cancer, it is obvious that most tumors are being diagnosed too late and there is at present no routinely used other modality able to detect earl-stage cancers, leading to a high mortality. Another example is lung cancer where low dose CT scans are being used in some countries for screening but the performance needs to be improved.
3. Your research suggests that liquid biopsy can detect minimal residual disease or recurrence months before traditional imaging. What implications does this early detection have for patient management—for example, could it enable low-toxicity “preventive” therapies to eliminate residual tumor cells—and what steps are needed to translate that vision into reality?
RE: Detection of minimal residual disease (MRD) has opened a new avenue for interventional clinical trials that assess which treatment is suitable to eliminate or control MRD (reviewed in Pantel & Alix-Panabieres, Nat. Rev. Clin. Oncol. 2025). These trials are needed to translate MRD diagnostic into reality. Promising data exist that de-escalation of toxic therapies such as chemotherapy in MRD-negative patients might become an option. In the EU/IHI project GUIDE.MRD (www.guidemrd-horizon.eu), we are currently assessing which ctDNA tests are best suitable for early detection of MRD and subsequent metastatic relapse in patients with colorectal, lung and pancreatic cancer. Additional assessment of the host response (e.g., tumor immunity) might be also important to predict the course of MRD towards overt metastasis.
- 29 Oct ,2025