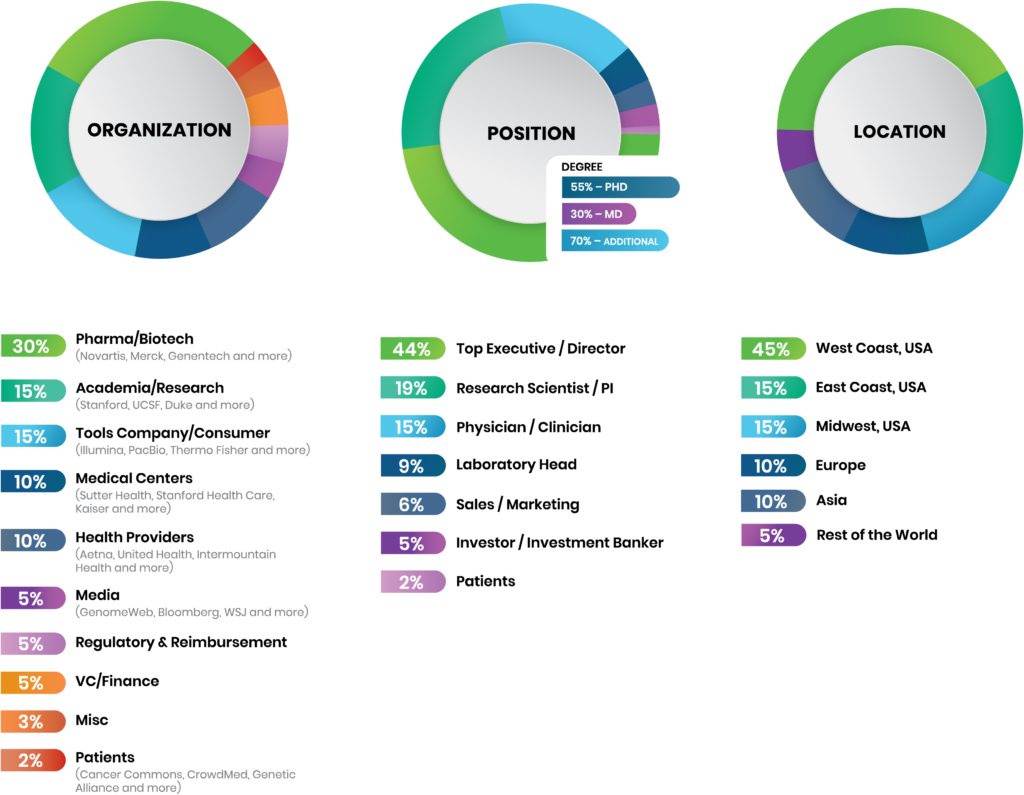
Multidisciplinary Attendee Breakdown
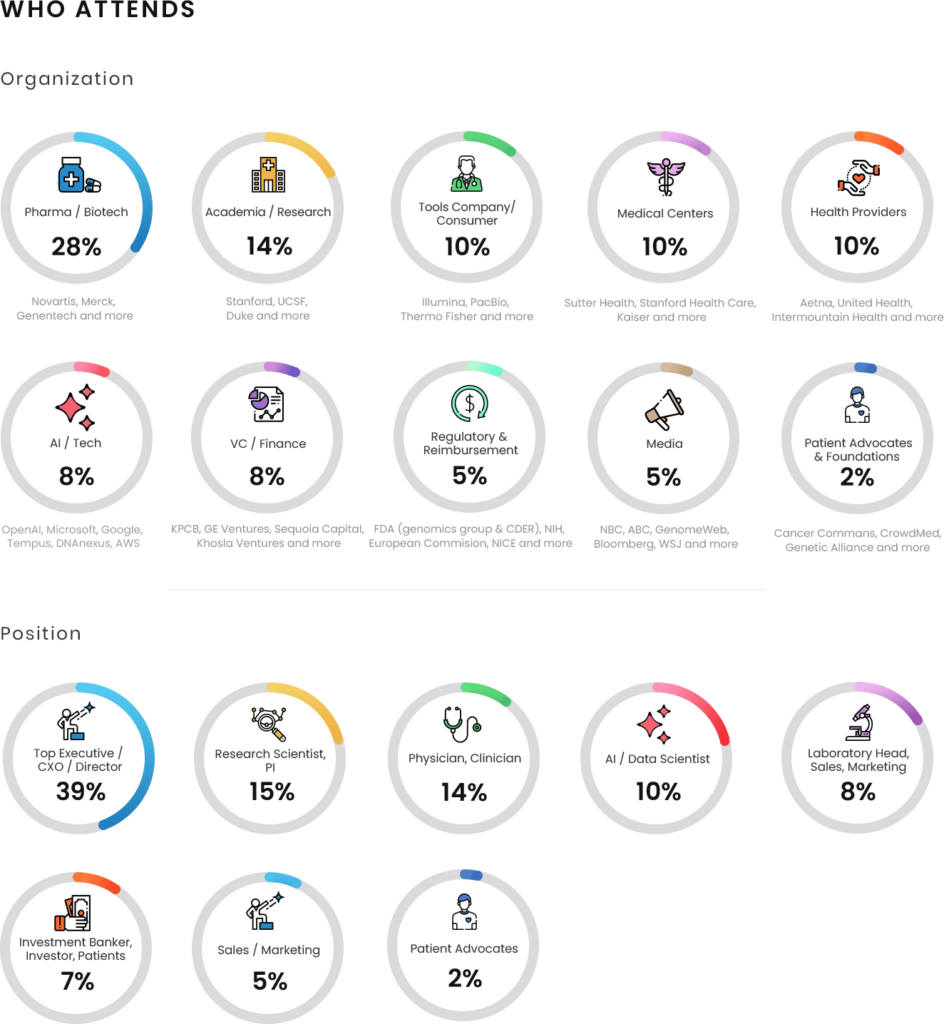
Who should Attend/Sponsor:
Track 1: Next-Gen Therapies
Topic Areas
- Engineered cell therapies: CAR-T/TCR/TIL/NK
- Allogeneic/off-the-shelf & in-vivo cell therapies
- Gene editing (CRISPR base/prime) & synthetic circuits
- Tumor microenvironment: biomarkers & combination strategies
- Personalized cancer vaccines & neoantigens
- Antibody-drug conjugates (ADCs) & targeted modalities
- RNA/oligo therapeutics: mRNA, siRNA, ASOs
- In-vivo gene therapy & myeloid programming
- CMC/GMP & scalable manufacturing/automation
- Clinical translation: trial design, resistance, access & IP
Who Attends
- Chief Medical Officers (CMO), Founders & Translational R&D Heads
- Oncology & Hematology Clinicians
- Immunology & Cell/Gene Therapy Scientists
- Clinical Development & Operations Leaders
- Manufacturing, CMC & Quality Executives
- Regulatory, Safety & Pharmacovigilance Directors
- Market Access, Reimbursement & Payer Executives
- Business Development & Alliance Management
- Venture & Innovation Investors & Business Strategy Execs
Who Should Sponsor
- CGT/editing platforms & vector/LNP delivery
- Cell processing, automation & CDMOs/CROs
- Potency assays, single-cell analytics & release testing
- GMP/MES/QMS/eBR software
- Cold-chain & logistics
- Regulatory/CMC & IP consultancies
- ADC & antibody engineering platforms
- Vaccine/neoantigen platforms
- CDx/biomarker partners & trial networks
- Patient support & access services
- Clinical-stage biotechs & manufacturing innovation startups
Track 2: AI & Computational Medicine
Topic Areas
- Foundation & multimodal models for medicine
- Imaging AI: radiomics/radiogenomics, contrast-free & low-dose
- Digital twins, predictive simulation & dose optimization
- Clinical decision support in EHR: integration & guardrails
- Real-world data pipelines & regulatory-grade RWE
- AI for trial design/eligibility & adaptive studies
- MLOps, governance, monitoring & safety
- Privacy-preserving/federated learning & security
- GenAI for molecules/proteins & target discovery
- Interpretability & validation from prediction to in-vivo
Who Attends
- Chief Digital & AI Officers (CDIO/CAIO)
- Chief Information Officers (CIO/CTO/CMIO)
- AI & Data Science Program Leads
- Radiology, Pathology & Clinical Informatics Directors
- Clinical Trial & RWE Strategy Executives
- Biostatistics & Machine Learning Leads
- Product, Engineering & MLOps Managers
- Payer Analytics & Outcomes Research Heads
- Digital Health Entrepreneurs & Innovation Leaders
- Academic Researchers in AI/ML for Medicine
- Investors & Corporate Strategy Executives
Who Should Sponsor
- Cloud/GPU/accelerator providers
- EHR/CDS & interoperability vendors
- Imaging vendors, PACS/VNA & edge devices
- Data platforms (FHIR/ETL/integration) & warehouses
- Model governance/monitoring/audit tools
- RWE/HEOR & trial analytics platforms
- Security, privacy, consent & identity providers
- Digital twin & simulation platforms
- AI-for-biopharma discovery & lab automation
- Annotation/curation & synthetic data providers
Track 3: Precision Diagnostics
Topic Areas
- Early detection & screening: MCED value & false-positive management
- MRD detection & monitoring: cfDNA, cfRNA, methylation, fragmentomics
- Therapy guidance: ctDNA-guided switching & response assessment
- Multi-omics liquid biopsy: exosomes/EVs, cfRNA & proteogenomics
- Integration with imaging & clinical biomarkers
- AI in diagnostics: assay design, interpretation & triage
- Pre-analytics, standardization & assay optimization
- Clinical validation, utility, coverage & reimbursement
- Regulatory pathways for early detection & MRD assays
- Population-scale screening & precision prevention
Who Attends
- Oncology, Pathology & Laboratory Medicine Chairs
- Clinical Diagnostics & Biomarker Development Leaders
- Medical & Scientific Directors of CLIA/CAP Labs
- Translational Science & Clinical Research Executives
- Bioinformatics & Computational Genomics Leads
- Payer & Health Technology Assessment (HTA) Leaders
- Regulatory Affairs & Quality Assurance Executives
- Diagnostics Business Development & Pharma Alliances
Who Should Sponsor
- MCED, MRD & liquid biopsy platforms
- Fragmentomics/methylation & cfRNA/EV technologies
- Sequencing (short/long-read) & library/sample prep
- AI diagnostics & decision-support software
- Bioinformatics, LIS/LIMS & workflow automation
- Reference labs & CLIA testing services
- CDx co-development & clinical trial assay partners
- Biobanking, pre-analytics & cold-chain
- Standards/QA & proficiency testing
- Reimbursement, market access & policy advisors
Track 4: Integrated Precision Medicine
Topic Areas
- Data factory: integrating EHR, imaging, labs & genomics
- Clinical-grade WGS/long-read & variant interpretation pipelines
- Population biobanks to bedside: cutting VUS & accelerating discovery
- Knowledgebases, curation & evidence frameworks
- Newborn, carrier screening & pharmacogenomics programs
- Learning health systems & regulatory-grade RWE
- Implementation at scale: governance, privacy, consent & data rights
- Reimbursement, payer partnerships & value-based models
- Equity, access & community implementation
- Precision aging & longevity: biomarkers, clocks, trials & digital endpoints
Who Attends
- Chief Medical & Scientific Officers (CMO/CSO)
- Chief Information, Innovation & Strategy Officers
- Genomic Medicine & Genetic Counseling Program Leaders
- Health-System Service Line & Implementation Executives
- Data Architecture, Bioinformatics & LIMS/LIS Directors
- Population Health & Precision Medicine Program Leads
- Payers, Value-Based Care & Quality Executives
- Geroscience & Longevity Research Leadership
Who Should Sponsor
- EHR & interoperability platforms
- Cloud data warehouses, analytics & data governance
- WGS/WES & long-read testing partners
- Variant knowledgebases, curation & interpretation tools
- LIMS/LIS, data catalog/metadata & lineage
- Consent, identity, privacy & security platforms
- Payers & value-based care partners
- Biobank/registry platforms & sample management
- Implementation/change-management consultancies
- Aging biomarkers, wearables & digital endpoint vendors